In Aug., 2019, State Administration for Market Regulation [1][2](hereinafter SAMR) approved 3 foods for special medical purposes (FSMP), bringing the total number of registered FSMPs to 35.
*Note: ChemLinked is based on the CFDA (a department under SAMR) database to ensure that the FSMP product has been successfully registered, while SAMR issues the relevant announcement before the database update.
| Company | Product Name | Formula Type | Age Scope | Country |
| Beingmate Co., Ltd | 昔倍护 | infant nutrition supplement | 0-12months | China |
| Pyeongtaek factory, Maeil Dairies Co., Ltd. | 爱思诺晨而慧 | infant premature or low birth weight formula food | 0-12months | South Korea |
| Pyeongtaek factory, Maeil Dairies Co., Ltd. | 爱思诺赋儿嘉 | infant lactose free formula food | 0-12months |
For the latest list of registered FSMPs, please visit F-list.
Foreign Enterprises Doing Well, South Korea Listed for the First Time
In China's FSMP's sector, oversea companies dominate, 25 out of the 35 registered FSMPs are produced by foreign enterprises, accounting for 71.4% of the total.
Dutch enterprises, which concentrate on infants FSMP have 6 registered formulae. Similarly, companies from Germany, Spain, and the US focus on infant use FSMP.
Infant Formulae Segmentation
According to the latest list, the number of FSMP infant formula has reached 25. From the chart, we can see that 10 out of 25 of those formulae are for premature or low birth weight infants. Additionally, considering some babies have trouble in digestion and absorption, some companies offer formulae with partially hydrolyzed milk protein, lactose free, or nutritional supplements (5, 4, 3 respectively).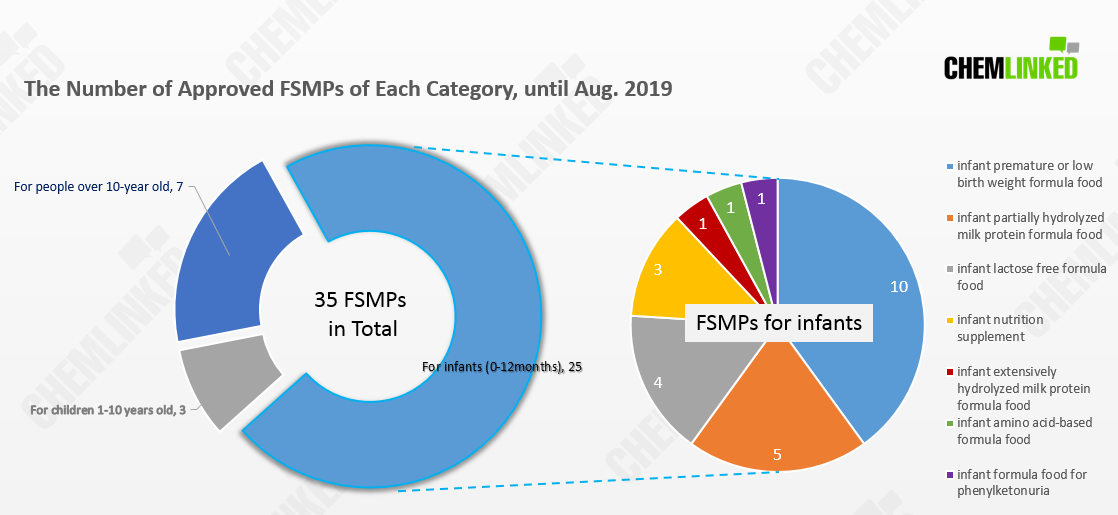
For more details (definition, registration process, etc.) about FSMP, please visit FSMP foodpedia.





 We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 








