FSMP registration in 2021H1
As revealed by SAMR, in the first half of 2021, 15 food for special medical purposes products were granted registration approval. All of them are domestic brands.
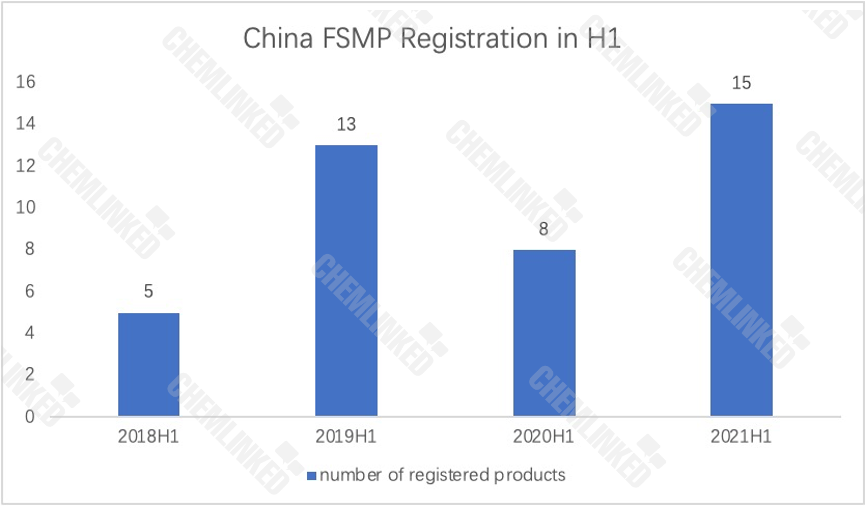

15 foods for special medical purposes products (FSMP) obtained registration approval in 2021H1. All of them are produced by domestic enterprises. By the end of June 2021, 72 FSMP products were registered in China. FSMP for infants accounted for 50% of all. Among them, premature/low birth weight infant formula, lactose free formula and partially-hydrolyzed lactoprotein infant formula occupy the majority.
As revealed by SAMR, in the first half of 2021, 15 food for special medical purposes products were granted registration approval. All of them are domestic brands.

 We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by food@chemlinked.com
We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by food@chemlinked.com

