During the period from June 13th to July 1st, State Administration for Market Regulation[1][2][3][4] (hereinafter SAMR) announced the approval of 8 food for special medical purposes (FSMP), which are listed below, bringing the total number of registered FSMP products to 32.
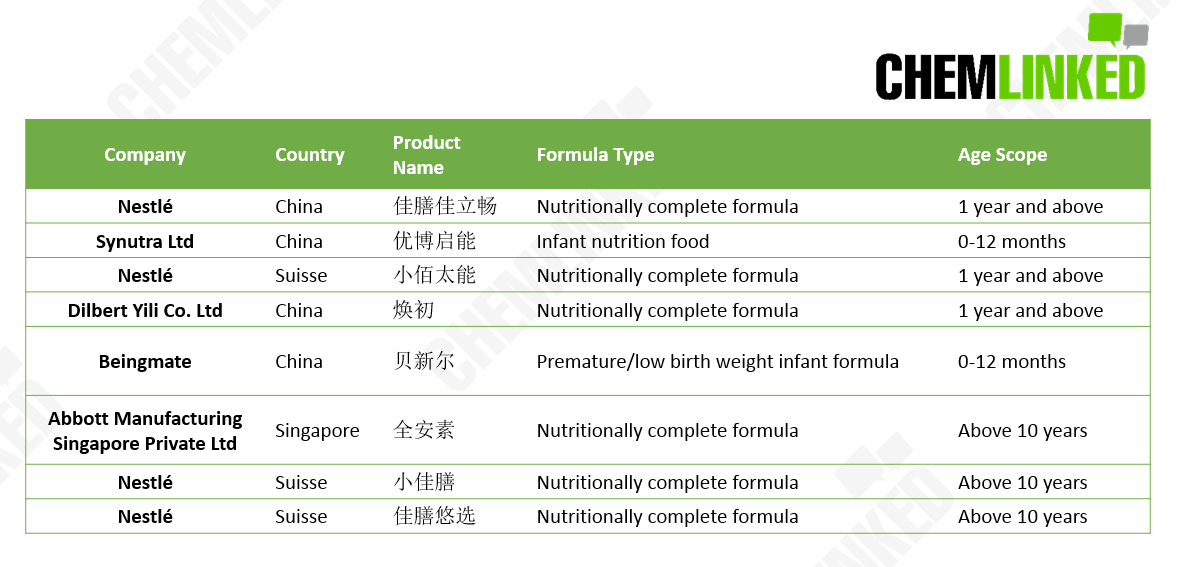
Overseas Formulae Account for Over 2/3 of all Registered Products
Despite half of the registered products announced in this notification being manufactured by Chinese domestic firms, the sector (in terms of registered products) is still dominated by international enterprise. 23 of the 32 registered FSMP products are manufactured by international enterprise. It is worth mentioning that some foreign brands such as Nestlé (Suisse SA company), Abbott (Singapore) which appeared in this notification have previously successfully registered products. The chart below shows the detailed number of registered FSMPs manufactured by different countries (statistics updated on July 3rd).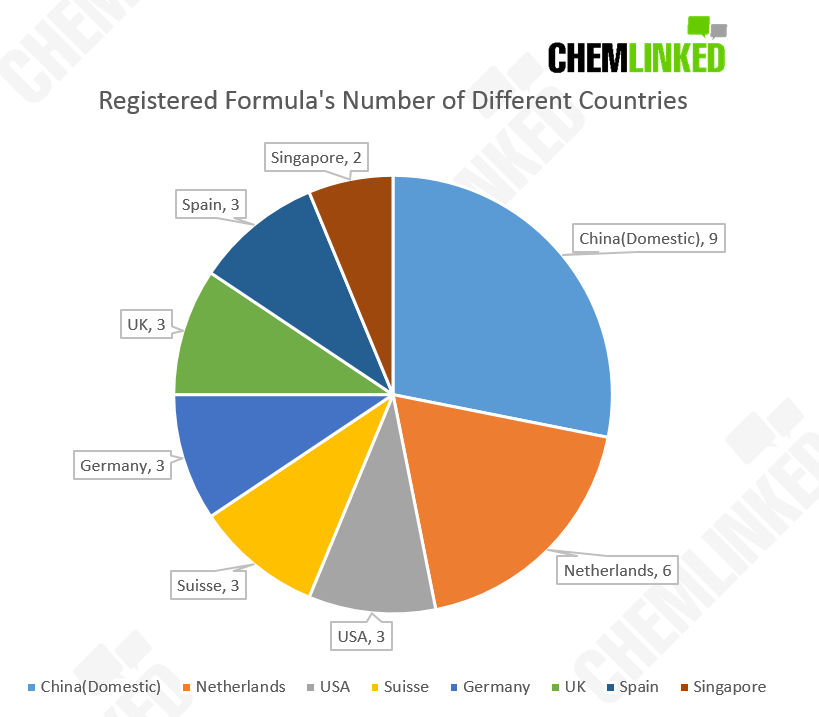
Infant Formulae Dominate
According to this SAMR announcement, an additional 2 infant formula foods were registered and the remaining 6 products are nutritionally complete formula foods, 4 out of 6 which were produced by Nestlé and another two products by Yili (China) and Abbott (Singapore) respectively.
Up to now, of the 32 registered FSMP products, 10 (=7 nutritionally complete + 3 incomplete + 0 specific full nutritional formula foods ) are designed for people aged 1 year old and above, and the remaining 22 formulas were manufactured for infants between 0-12 months. The chart shown below is the number of registered products of each category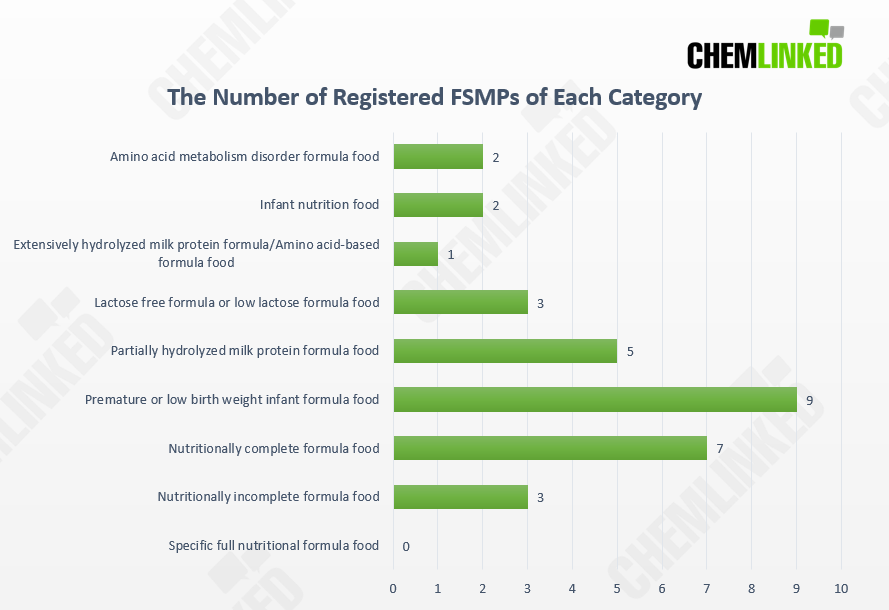





 We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 









