Take home
- Both domestic and overseas infant formula manufacturers can only market “3 product lines (3 stages per product line) in China.
- The registration rule will take effect as of 1 Oct 2016.
On 8 Jun 2016, China CFDA issued the long-awaited “Administrative Measures for Registration of Infant and Young Children Milk Powder Formula Recipes”. Its finalization is a major step in China’s reform of its IF industry. The important reforms include
- CNCA registration of overseas IF manufacturers
- Relicensing of domestic IF manufacturers
- CFDA registration of domestic and imported IF recipes
- Promotion of merger & acquisition of IF enterprises.
Restricting the number of brands and recipes is controversial and a major source of concern for many in the industry. China has 103 licensed infant formula manufacturers which market around 2,000 different products. The large product portfolio, differential labeling of identical formulations and region specific marketing of these products has made it a nightmare for Chinese consumers and regulators. By implementing the extremely strict new rule, China will push IF companies to develop their R&D ability, upgrade production conditions and promote product heterogeneity.
The new rule contains 6 chapters (general provisions, application and registration, labels and instructions, supervision and administration, legal responsibilities and supplementary provisions) totaling 49 articles. It’s quite similar to its previous draft for WTO nortification but more specified.
Applicant
Approved domestic and foreign IF manufacturers should be responsible for applying for registration of IF recipes with CFDA. In addition to pre-market approval application, they should test every batch of IF products shipped out of the factories.
3 series, 9 formulations
There must be significant difference between recipes for the same stage applied by the same company, supported by solid scientific evidence. Every manufacturer can only hold 3 series and 9 recipes at most. Every series should include infant formula (0-6 months, stage 1), formula for elder infants (6-12 months, stage 2) and formula for young children stage 1 (12-36 months, stage 3).
Wholly-owned subsidiaries who have already had approved IF recipes and been licensed for production are allowed to use other recipes registered by other wholly-owned subsidiaries under the same group company.
Registration dossier
- Application form;
- Quality safety standards for raw and auxiliary materials;
- R&D report for IF recipe;
- Production process description;
- Test report;
- Supporting documents for proving R&D, production and testing abilities;
- Other supporting documents for demonstrating the science and safety of the recipe;
Meanwhile, the applicant should also submit the copy of product label and instructions as well as description and supporting materials for claims made.
CFDA will issue supporting documents for the registration rule later to specify requirements for preparing above dossier.
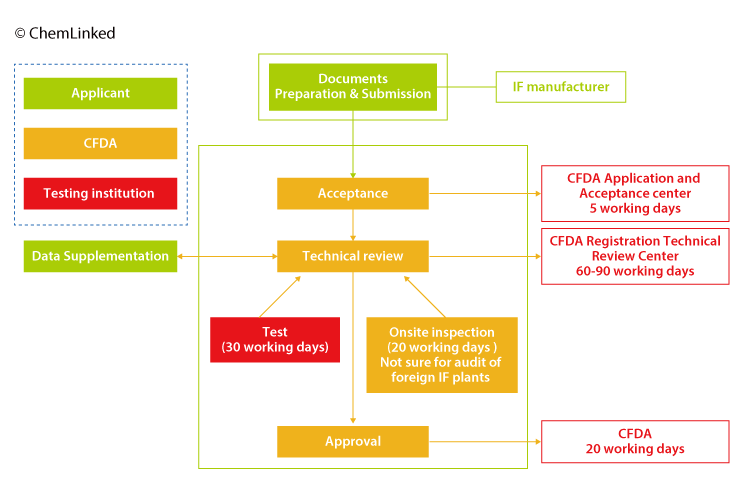
Registration procedure & timeframe
More specific and stricter requirements for infant formula labeling
Infant formula enterprises probably need to adjust all of their existing labels according to the new requirements.
- If a product name includes animal source like goat or cow, the animal source of raw materials such as raw milk, milk powder and whey protein should be indicated in the ingredient list as well. If raw materials used are derived from two animals, the percentage of raw materials from cow and goat should be marked in the ingredient list.
- In the ingredient list, edible vegetable oils used should be listed in descending order of weight;
- The nutrients in the nutrition information table on the label should be listed in the order appeared in the national standards GB 10765-2010 and GB 10767-2010, categorized by energy, protein, fat, carbohydrate, vitamins, minerals, optional ingredients, etc.
- If a label claims the source of raw milk or milk powder, the country of origin should be specified instead of using blurring terms like “imported milk source”, “from foreign ranch”, “environmentally friendly ranch”, “imported raw materials”, etc.
Grace period?
For CBEC infant formula, it’s already specified by the Ministry of Finance that the deadline for selling unregistered IF products is 1 Jan 2018. For general trade, the buffer time is probably the same but still needed to be confirmed by CFDA.
Reference Link
- CFDA press release
- Administrative Measures for Registration of Infant and Young Children Milk Powder Formula Recipes (English version prepared by ChemLinked)




 We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 






