On November 28, 2023, Health Canada announced to seek public comments on a proposal to modernize the regulations for foods for special dietary use (FSDU) and infant foods, which are currently regulated by the Divisions 24 and 25 of the Food and Drug Regulations. Any comments can be sent to bns-bsn@hc-sc.gc.ca until February 6, 2024.
1. Background and purpose
The current regulatory framework for FSDU and infant foods were established decades ago and has undergone few changes. With the development of the food industry, many problems of the current regulatory framework begin to expose, such as the narrow application scope, the inflexible and outdated compositional and labelling requirements, as well as the misalignment with international legislations. These issues pose challenges for innovative products to enter the Canadian market, leading the country with a less diversified supply layout where may be more severely affected by the possible shortage.
This proposal aims to get rid of these limits, while supporting increased innovation, improving alignment with international jurisdictions, and reducing barriers to the entry of these foods into Canada.
2. Product classification
Under the current framework, both FDSU and infant foods are comprised of three categories. After reintegrating these categories, the newly proposed framework sets two new divisions, namely, foods for a special dietary purpose (FSDP), and other foods. The term "FSMP" will be used to cover several key products used to fall under FSDU and infant foods, including infant formula, prepackaged human milk, medical foods for infants (including HMF), medical foods for ages one and more, and total diet replacements for weight reduction.
Besides, "foods containing infant formula as an ingredient" are eliminated from the regulatory framework, since there are no such products on the market.
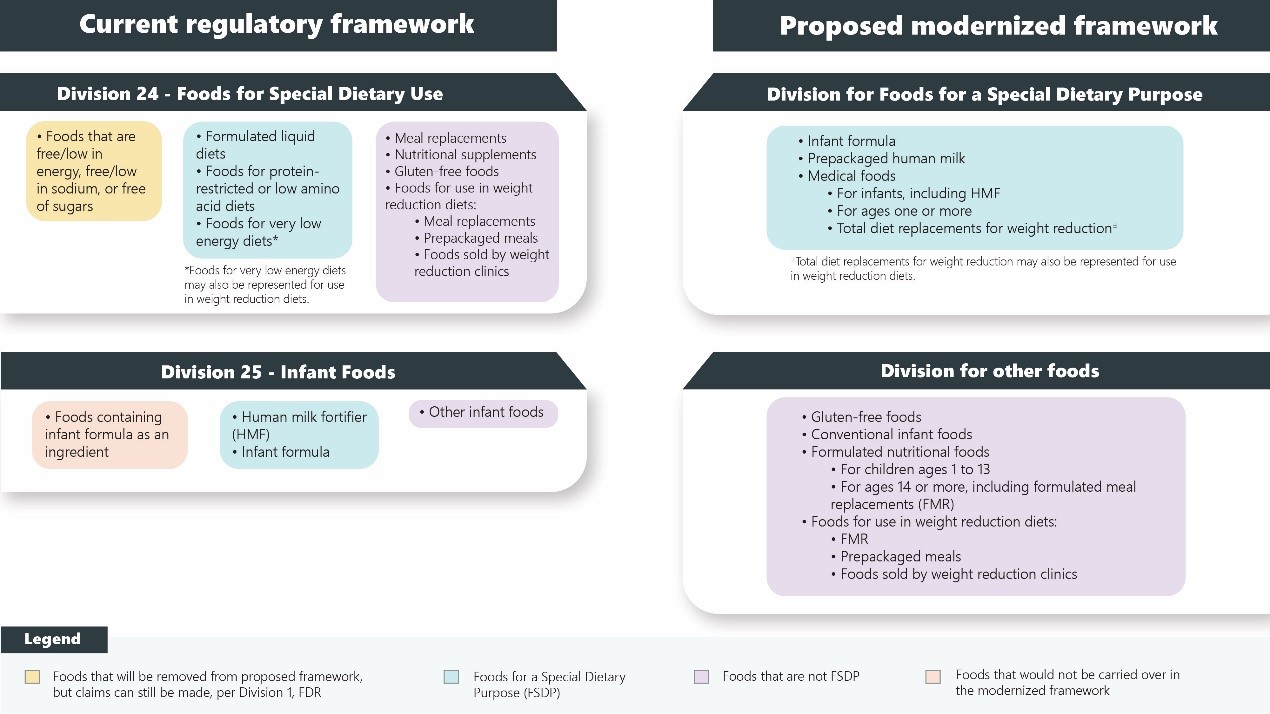 Comparison of food classifications between the two regulatory frameworks Source: Health Canada
Comparison of food classifications between the two regulatory frameworks Source: Health Canada Comparison of FSDU and FSDP Source: Health Canada
Comparison of FSDU and FSDP Source: Health Canada
3. New regulatory requirements
The draft establishes new requirements for each category under FSDP and other foods within the proposed regulatory framework. These requirements cover product definition, composition, labeling, claims, premarket authorization, advertising restrictions, etc. Take infant formula for example:
Item | Description/Requirements |
Definition | Any food that is labelled or advertised for use as a partial or total replacement for human milk and is intended for consumption by infants. |
Composition |
|
Labeling |
a) Information on the principal display panel, such as the age range of the intended product user; b) Directions for use, including the preparation, storage and disposal; c) Lot numbers; d) Information on protecting and promoting breastfeeding; e) Cautionary statements; f) Prohibition on cross branding; g) Labeling exemptions; h) Changes to labeled text or images. |
Claims |
|
Premarket authorization |
|
Advertising restrictions |
|
In addition to the proposed category-specific regulatory requirements, the draft specifies some other regulatory requirements applicable to all FSDP, including shortage provisions, stop-sale provisions, clinical trial and remarket review requirements.





 We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 










