On June 5, 2023, ChemLinked’s parent company REACH24H Consulting group successfully helped clients get four import feed registration certificates for four types of feed additive mixture, approved from China's Ministry of Agriculture and Rural Affairs (MoARA).
MoARA Notice No. 675: 178 imported feed and feed additive products from 98 companies have obtained or extended the registration

According to Administrative Measures for Imported Feed and Feed Additives Registration, before selling and/or using feed and/or feed additive products in China, overseas companies must entrust its permanent representative office in China or an agent company in China to obtain an import registration certificate. This requirement is applicable to single feed, feed additive premix, concentrated feeds, compound feeds, supplementary concentrate, feed additive, feed additive mixture, compound pet feed (for dogs and cats), and pet feed additive premix. Failure to obtain the certificate may result in the failure to enter the Chinese market.
To accelerate the application process and avoid unnecessary loss, ChemLinked compiles the procedure and tips as below.
1. Document Preparation
When applying for the registration, overseas companies must prepare the following documents.
No. | Requirements | For the application of regular products | For the application of novel feed/feed additives |
1 | Contents | √ | √ |
2 | Application form for import feed and/or feed additive | √ | √ |
3 | Qualification documents of the representative office/ domestic agent company | √ | √ |
4 | Authorization letter | √ | √ |
5 | Proof to indicate the product is approved to be produced and used at the origin country | √ | √ |
6 | Physicochemical properties | √ | √ |
7 | Product origin and composition | √ | √ |
8 | Manufacturing process | √ | √ |
9 | Quality standard and testing methods | √ | √ |
10 | Labels used in the origin country, label template in Chinese and trademarks | √ | √ |
11 | Usage purpose, application scope and usage method | √ | √ |
12 | Packaging materials, specification, shelf life and storage conditions | √ | √ |
13 | Registration and application status in other countries and regions other than the origin country | √ | √ |
14 | Identification report of active ingredient’s chemical structure; or identification report of the taxonomy of animal, plant and microorganisms | × | √ |
15 | Test report of product efficacy and safety evaluation | × | √ |
16 | Report of human health impact analysis | × | √ |
17 | Test report of product stability and environmental impact | × | √ |
18 | Testing method for the Maximum Residue Limit (MRL) and effective components in feed products | √ | |
19 | Major references | × | √ |
Tips: In practice, due to the diverse sources and complex composition of products, it is very easy to encounter problems regarding product’s quality standard, hygiene indicators, and product testing. Moreover, some overseas companies may develop a certain product specifically for China, without being approved in the country of origin, which also leads to the failure of getting registered in China.
Therefore, REACH24H advises companies to fully understand the legal requirements of the country of origin and China, then determine product's characteristics based on its main and active ingredients, production process, target animal and usage amount, and prepare application materials with targeted focus to increase the success rate of application. |
2. Declaration Procedure for Import Registration Certificate
Once the application materials are ready, companies can submit their applications through their permanent representative office in China or an agent company in China. Products not listed in the "Catalogue of Feed Raw Materials" or the "Catalogue of Feed Additive Varieties" require expert review by the National Feed Review Committee before undergoing the quality re-inspection, which is an additional step comparing to products listed in the two catalogues.
Picture 1: Application procedure for regular feed and feed additive
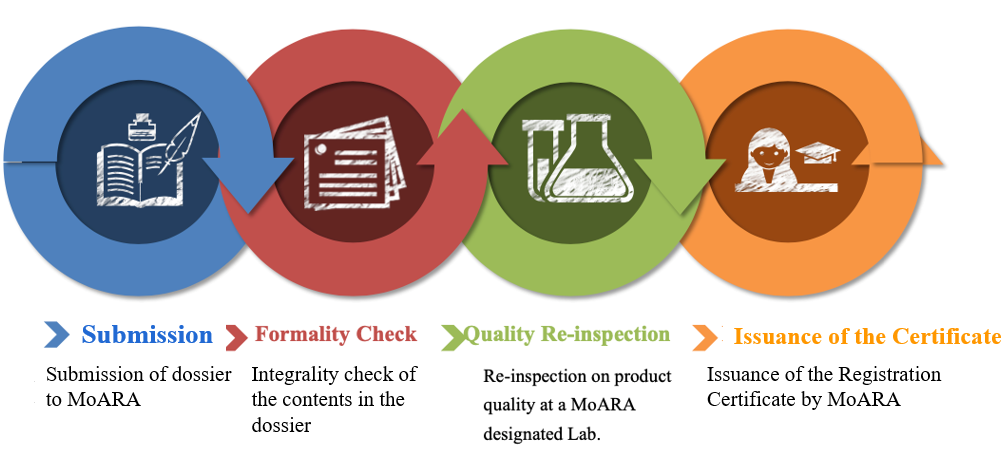
Picture 2: Application procedure for novel feed and feed additive

Tips: The following are common reasons for failed applications. Enterprises shall pay high attention to them.
|
Our Service
Due to various trade and regulatory barriers between the country of origin and the export destination, entrusting an experienced agency like REACH24H is necessary to save time and effort, and avoid pitfalls during the application process. For registration or consultation service, please feel free to email contact@chemlinked.com.





 We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by
We provide full-scale global food market entry services (including product registration, ingredient review, regulatory consultation, customized training, market research, branding strategy). Please contact us to discuss how we can help you by 










